A Better Breathalyzer
About Optica
08 October 2013
A Better Breathalyzer
FOR IMMEDIATE RELEASE
Contact:
Lyndsay Meyer
The Optical Society
+1.202.416.1435
lmeyer@osa.org
A Better Breathalyzer
Gel-filled gemstones make new reusable, color-changing tool for detecting alcohol vapor concentration

Red Light, Green Light: The opal-based alcohol sensor is green before use (top) but turns redder in the presence of alcohol vapor (bottom).
Credit: Riccardo Pernice, Università degli Studi di Palermo
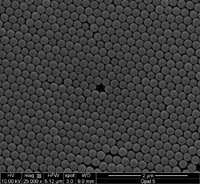
A closeup of the surface of the opal, taken with a scanning electron microscope (SEM). The ethanol-responsive gel used in the device would fill the spaces between the rows of regularly spaced nanoparticles that comprise the opal.
Credit: Riccardo Pernice, Università degli Studi di Palermo
WASHINGTON, Oct. 8, 2013—To gauge whether suspects involved in accidents or routine traffic stops have been driving drunk, police officers pair field sobriety tests with breathalyzers, which signal the presence of alcohol in the breath. Most breathalyzers are expensive and unable to test for precise concentrations of alcohol. Offering a better solution, Italian researchers have developed a novel idea for an inexpensive, portable breathalyzer whose color would change from green to red with higher alcohol concentrations. But unlike current color change-based devices, this sensor would be reusable and could also provide a precise digital readout.
The new design is the first to use the sensing properties of opals, a type of gemstone, to detect the gas version of ethanol, the intoxicating component of commercial liquor, by inducing a change in color that is visible to the human eye. The research team describes their new method in a proof-of-concept paper published today in The Optical Society’s (OSA) journal Optical Materials Express.
The portable breathalyzers preferred by roadside police use expensive electronic readouts, but these devices lack the “immediate and intuitive” color change that tells police whether the alcohol content of a suspect’s breath puts them in the legal red zone, said first author Riccardo Pernice of the Università degli Studi di Palermo in Italy. Techniques that do use color change to assess the level of alcohol concentration are typically less expensive, but they cannot give a precise reading of the alcohol concentration and most are use-once-and-toss. Pernice said his team’s proposed device combines the best elements of each of these two breathalyzer models.
“Our approach enables an optical, naked-eye detection as a color change from green to red, like litmus paper,” Pernice said. “But it also potentially permits accurate quantitative measurements” with the addition of an electronic system or a color detector.
The method is inspired by the natural behavior of opals, gemstones whose iridescence illustrates their ability to manipulate light. Scientists use manufactured versions of opals and other photonic crystals to detect acidity or the presence of liquid ethanol, but until now little attention has been paid by researchers to detecting gaseous ethanol, the researchers said.
In their new setup, the researchers created sheets of manufactured opal about one centimeter square and just a few hundred billionths of a meter thick, as thin as some of the films on soap bubbles. The opals are pumped full of a gel tuned to respond to ethanol vapor. At increasing ethanol concentrations, the gel swells, changing the way light travels through the gel-filled opal and causing the sample to become red.
The change in color is clearly visible to the naked eye, Pernice said and the device is usable multiple times. After performing the measurements, researchers found that the sample gradually regained its original green color after less than one minute of exposure in air. He added that the sensor does not pose environmental concerns for disposal after use, since it is made of all non-toxic materials, and that it also does not react to acetone, one of the many substances that can be falsely identified as ethanol by some breath machines.
The device is currently able to detect alcohol at much higher concentrations compared to other portable alcohol sensors. In the coming months, the researchers hope to explore the device’s use at lower concentrations as well.
Paper: "Opals infiltrated with a stimuli-responsive hydrogel for ethanol vapor sensing," R. Pernice et al., Optical Materials Express, Vol. 3, Issue 11, pp. 1820-1833 (2013).
###
About Optical Materials Express
Optical Materials Express (OMEx) is OSA's newest peer-reviewed, open-access journal focusing on the synthesis, processing and characterization of materials for applications in optics and photonics. OMEx, which launched in April 2011, primarily emphasizes advances in novel optical materials, their properties, modeling, synthesis and fabrication techniques; how such materials contribute to novel optical behavior; and how they enable new or improved optical devices. For more information, visit www.OpticsInfoBase.org/OMEx.
About OSA
Founded in 1916, The Optical Society (OSA) is the leading professional society for scientists, engineers, students and business leaders who fuel discoveries, shape real-world applications and accelerate achievements in the science of light. Through world-renowned publications, meetings and membership programs, OSA provides quality research, inspired interactions and dedicated resources for its extensive global network of professionals in optics and photonics. For more information, visit www.osa.org.

